A delivery system is an engineered nanotechnology that allows the release of a therapeutic agent WHERE, HOW and WHEN it should be.
DRUG DESIGN
During the earliest phases of drug development, it is key to develop a thorough understanding of the movement of the compound of interest through the ADME (absorption, distribution, metabolism and excretion) as well as toxicity and efficacy profiling of the compound. This will help to reduce the possibilities of unexpected results in later phases of the development and failure to pass regulatory requirements.
The use of a drug delivery system can effectively benefit the pharmacokinetics and pharmacodynamics of said agent.
DELIVERY PROCESS AND MECHANISM
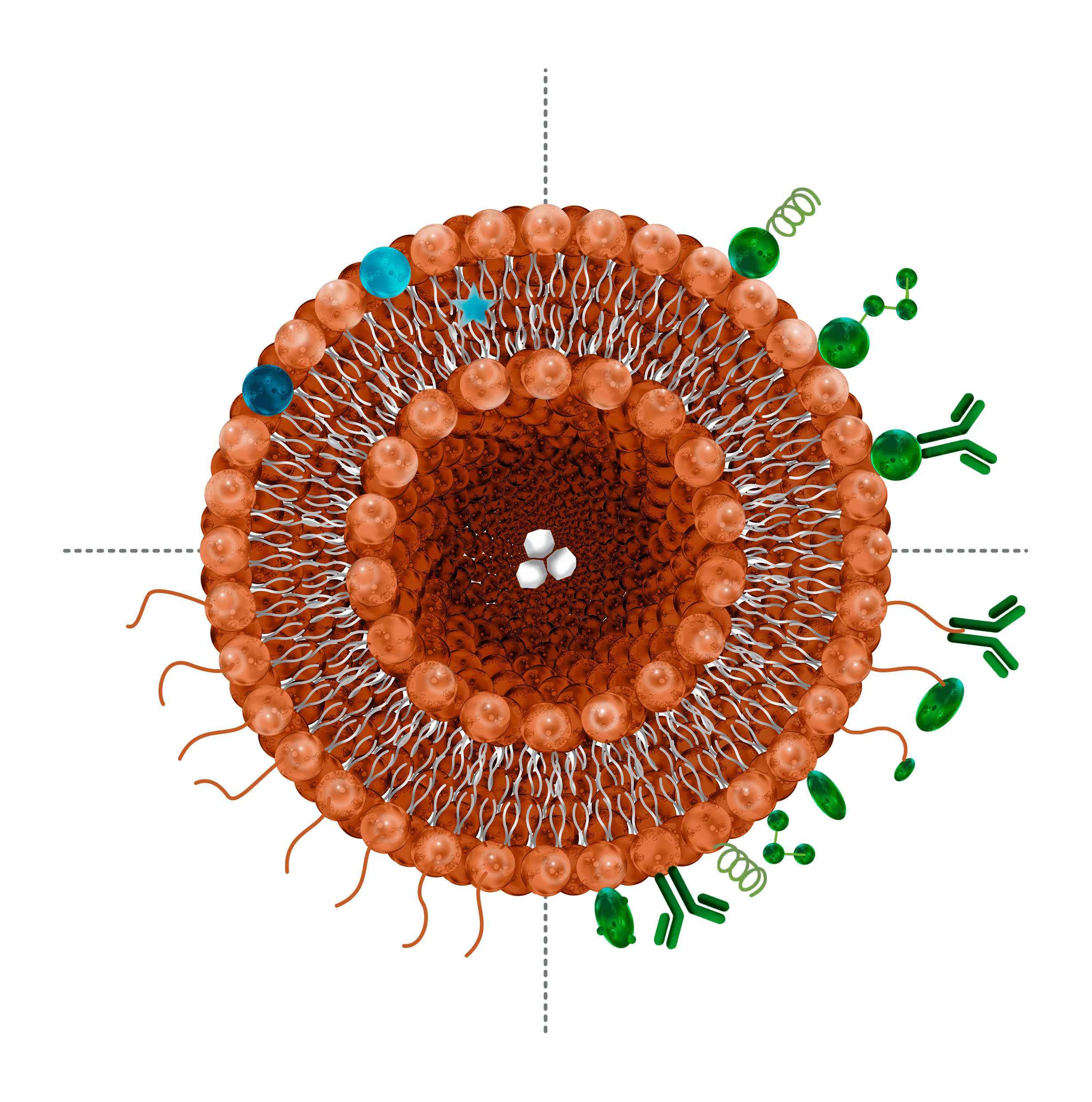
Load and protect
A nanosystem is a stable and tunable nanoscale structure. In nanopharmaceutics, they are used as a vehicle to transport compounds along the body in a more efficient way. The compound of interest can be either encapsulated within, like in liposomes, synthetic exosomes and polymeric nanoparticles or attached to its surface like in metallic nanoparticles and polymeric nanoparticles. This enhances the solubility of the drug and protects it from damaging external agents like oxygen. All the characteristics required need to be tested to ensure the future behaviour of the nanocapsule.
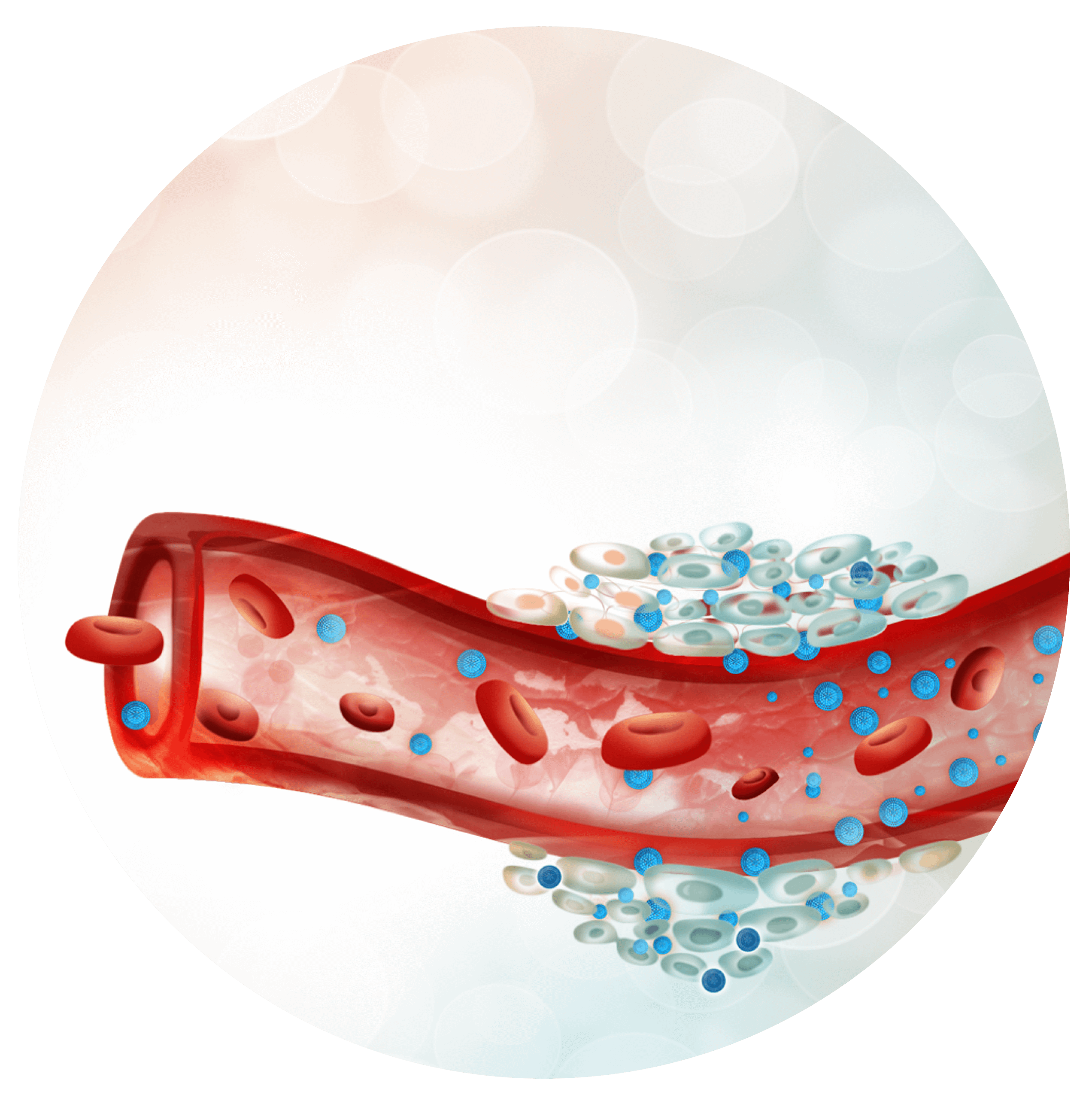
Deliver to the right place
Now that the compound is loaded in a nanocarrier, once administered its behaviour will be the nanosystem’s, allowing the developer to control better the substance pharmacokinetics. Time of circulation can be modified, and non-specific interactions avoided thanks to a proper coating. This also makes possible other administration routes thanks to an increase in solubility and protection against gastrointestinal conditions. Reaching difficult targets will be easier, allowing to overcome the blood brain barrier to treat or diagnose central nervous system diseases. Additionally, using a specific strategy the nanocarrier can be tracked with non-invasive imaging techniques to get to know its ADME profile or to detect disease biomarkers as a diagnosis method. Finally, and most importantly, the nanovehicle will accumulate in the active site reducing toxicity problems
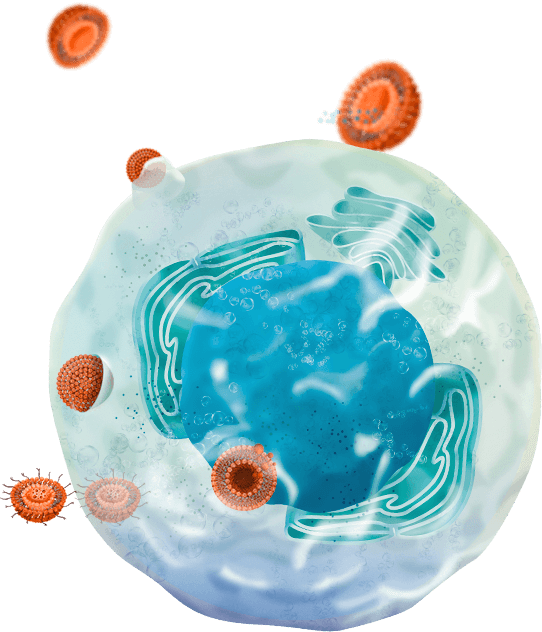
Release in a controlled manner
Finally, the nanocarrier has arrived at its destination, where it should release the pharmaceutical substance that it has been protecting all the way there. This destination could be defined as the bloodstream, a specific tissue, or even the cytoplasm or an organelle in a targeted cell type. Several strategies can be used to control this precisely the release settings required. The pace of release, the entryway desired, the release in response to a physico-chemical stimulus are just some methods that can be used.
APPLICATIONS
Molecular therapy
Wound healing
Cancer therapy
Ocular drug delivery
Brain drug delivery
Stem cell tracking
Bio-sensing
Photothermal therapy
Immunomodulating response
Tissue engineering
Vaccines
Oral insulin administration

WHAT CAN BE ACHIEVED
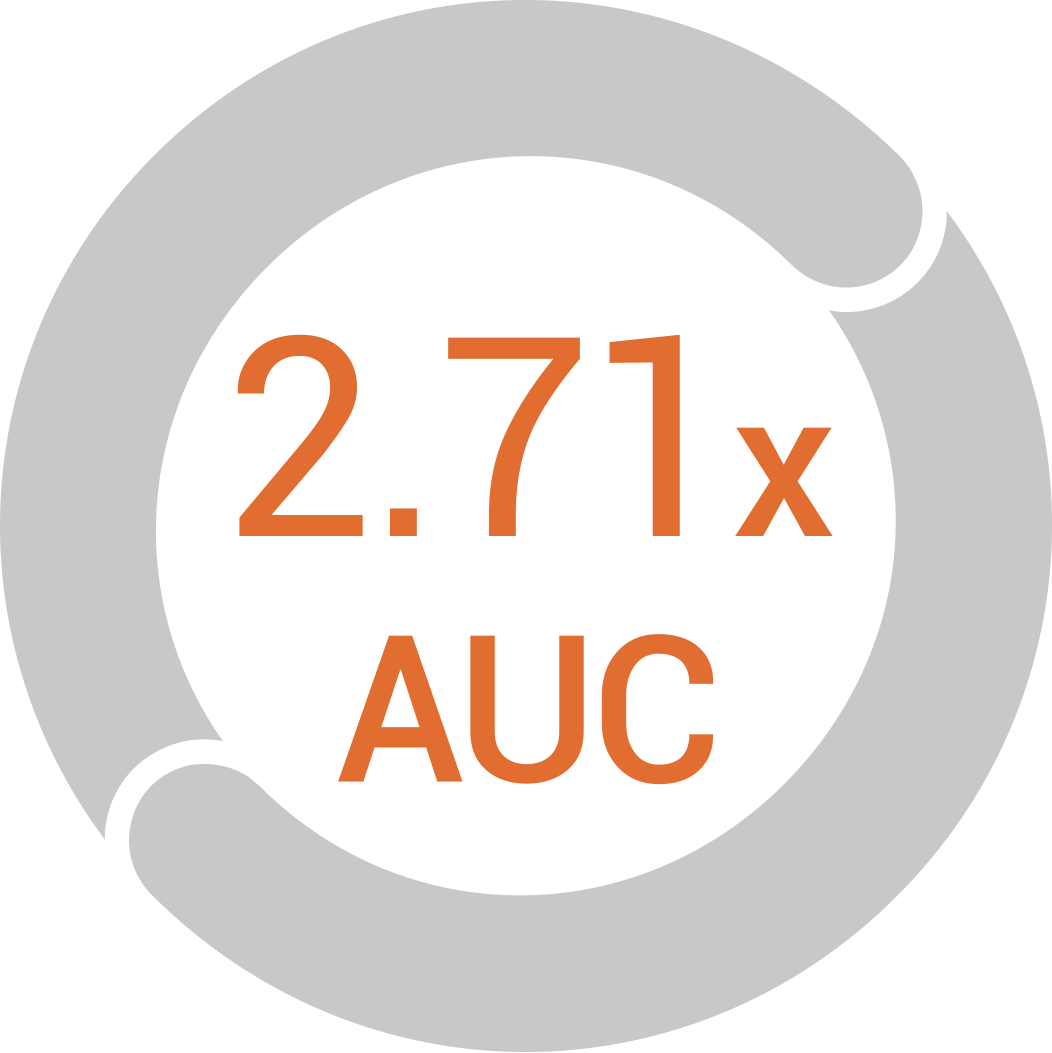
The oral bioavailability of the
liposomed drug at least doubles
in in vivo studies compared to its
free form*
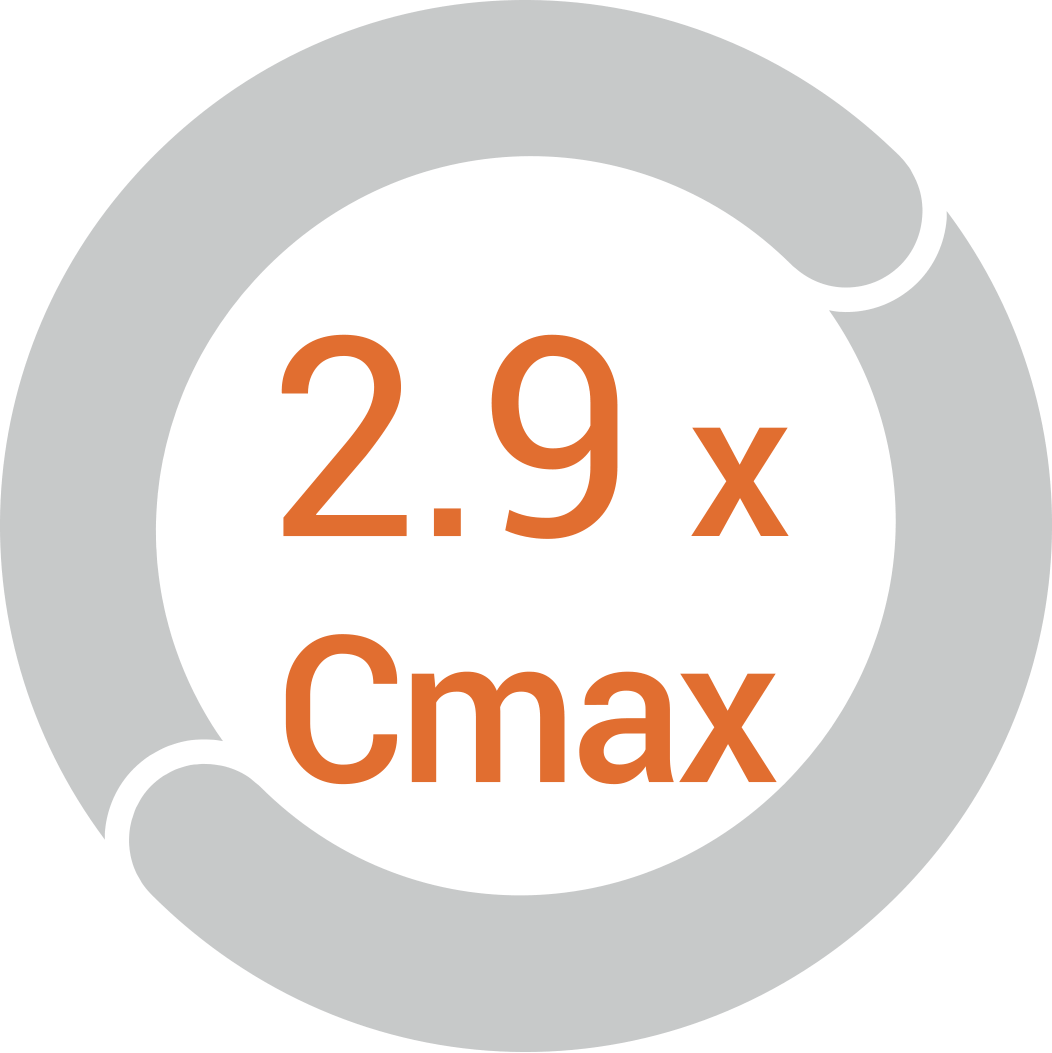
The concentration of liposomed
drug in the targeted area almost
triples in in vivo studies compared
to its free form*

Up to 5 h of delay in the
maximum concentration peak
time for sustained release
delivery systems*
* References
Bagre, A.P., Jain, K., Jain, N.K., 2013. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. International Journal of Pharmaceutics 456, 31–40. https://doi.org/10.1016/j.ijpharm.2013.08.037
Brocks, D.R., Betageri, G.V., 2010. Enhanced oral absorption of halofantrine enantiomers after encapsulation in a proliposomal formulation. Journal of Pharmacy and Pharmacology 54, 1049–1053. https://doi.org/10.1211/002235702320266190
Chen, H., Wu, J., Sun, M., Guo, C., Yu, A., Cao, F., Zhao, L., Tan, Q., Zhai, G., 2012. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. Journal of Liposome Research 22, 100–109. https://doi.org/10.3109/08982104.2011.621127
Chen, Y., Lu, Y., Chen, J., Lai, J., Sun, J., Hu, F., Wu, W., 2009. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. International Journal of Pharmaceutics 376, 153–160. https://doi.org/10.1016/j.ijpharm.2009.04.022
Chu, C., Tong, S., Xu, Y., Wang, L., Fu, M., Ge, Y., Yu, J., Xu, X., 2011. Proliposomes for oral delivery of dehydrosilymarin: preparation and evaluation in vitro and in vivo. Acta Pharmacol Sin 32, 973–980. https://doi.org/10.1038/aps.2011.25
Deng, J., Zhang, Z., Liu, C., Yin, L., Zhou, J., Lv, H., 2015. The studies of N-Octyl-N-Arginine-Chitosan coated liposome as an oral delivery system of Cyclosporine A. Journal of Pharmacy and Pharmacology 67, 1363–1370. https://doi.org/10.1111/jphp.12448
El-Zein, H., Riad, L., El-Bary, A.A., 1998. Enhancement of carbamazepine dissolution: in vitro and in vivo evaluation. International Journal of Pharmaceutics 168, 209–220. https://doi.org/10.1016/S0378-5173(98)00093-3
Ghassemi, S., Haeri, A., Shahhosseini, S., Dadashzadeh, S., 2018. Labrasol-Enriched Nanoliposomal Formulation: Novel Approach to Improve Oral Absorption of Water-Insoluble Drug, Carvedilol. AAPS PharmSciTech 19, 2961–2970. https://doi.org/10.1208/s12249-018-1118-9
Jain, S., Kumar, D., Swarnakar, N.K., Thanki, K., 2012. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials 33, 6758–6768. https://doi.org/10.1016/j.biomaterials.2012.05.026
Janga, K.Y., Jukanti, R., Velpula, A., Sunkavalli, S., Bandari, S., Kandadi, P., Veerareddy, P.R., 2012. Bioavailability enhancement of zaleplon via proliposomes: Role of surface charge. European Journal of Pharmaceutics and Biopharmaceutics 80, 347–357. https://doi.org/10.1016/j.ejpb.2011.10.010
Kim, J.H., Shin, D.H., Kim, J.-S., 2018. Preparation, characterization, and pharmacokinetics of liposomal docetaxel for oral administration. Arch. Pharm. Res. 41, 765–775. https://doi.org/10.1007/s12272-018-1046-y
Kovvasu, S.P., Kunamaneni, P., Yeung, S., Rueda, J., Betageri, G.V., 2019. Formulation of Dronedarone Hydrochloride-Loaded Proliposomes: In Vitro and In Vivo Evaluation Using Caco-2 and Rat Model. AAPS PharmSciTech 20, 226. https://doi.org/10.1208/s12249-019-1437-5
Li, C., Zhang, Su, Feng, Long, Chen, 2012. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. IJN 5995. https://doi.org/10.2147/IJN.S38043
Nekkanti, V., Rueda, J., Wang, Z., Betageri, G.V., 2016a. Comparative evaluation of proliposomes and self micro-emulsifying drug delivery system for improved oral bioavailability of nisoldipine. International Journal of Pharmaceutics 505, 79–88. https://doi.org/10.1016/j.ijpharm.2016.03.065
Nekkanti, V., Rueda, J., Wang, Z., Betageri, G.V., 2016b. Design, Characterization, and In Vivo Pharmacokinetics of Tacrolimus Proliposomes. AAPS PharmSciTech 17, 1019–1029. https://doi.org/10.1208/s12249-015-0428-4
Shah, N.M., Parikh, J., Namdeo, A., Subramanian, N., Bhowmick, S., 2006. Preparation, Characterization and In Vivo Studies of Proliposomes Containing Cyclosporine A. j nanosci nanotechnol 6, 2967–2973. https://doi.org/10.1166/jnn.2006.403
Shen, Y., Ji, Y., Xu, S., Chen, D. quan, Tu, J., 2011. Multivesicular liposome formulations for the sustained delivery of ropivacaine hydrochloride: Preparation, characterization, and pharmacokinetics. Drug Delivery 18, 361–366. https://doi.org/10.3109/10717544.2011.557788
Sugihara, H., Yamamoto, H., Kawashima, Y., Takeuchi, H., 2012. Effectiveness of submicronized chitosan-coated liposomes in oral absorption of indomethacin. Journal of Liposome Research 22, 72–79. https://doi.org/10.3109/08982104.2011.621128
Telange, D.R., Patil, A.T., Pethe, A.M., Fegade, H., Anand, S., Dave, V.S., 2017. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. European Journal of Pharmaceutical Sciences 108, 36–49. https://doi.org/10.1016/j.ejps.2016.12.009
Velpula, A., Jukanti, R., Janga, K.Y., Sunkavalli, S., Bandari, S., Kandadi, P., Veerareddy, P.R., 2013. Proliposome powders for enhanced intestinal absorption and bioavailability of raloxifene hydrochloride: effect of surface charge. Drug Development and Industrial Pharmacy 39, 1895–1906. https://doi.org/10.3109/03639045.2012.670641
Wang, Q., Liu, W., Wang, J., Liu, H., Chen, Y., 2019. Preparation and Pharmacokinetic Study of Daidzein Long-Circulating Liposomes. Nanoscale Res Lett 14, 321. https://doi.org/10.1186/s11671-019-3164-y
Wang, X., Fan, J., Liu, Y., Zhao, B., Jia, Z., Zhang, Q., 2011. Bioavailability and pharmacokinetics of sorafenib suspension, nanoparticles and nanomatrix for oral administration to rat. International Journal of Pharmaceutics 419, 339–346. https://doi.org/10.1016/j.ijpharm.2011.08.003
Xu, H., He, L., Nie, S., Guan, J., Zhang, X., Yang, X., Pan, W., 2009. Optimized preparation of vinpocetine proliposomes by a novel method and in vivo evaluation of its pharmacokinetics in New Zealand rabbits. Journal of Controlled Release 140, 61–68. https://doi.org/10.1016/j.jconrel.2009.07.014
Yanamandra, S., Venkatesan, N., Kadajji, V.G., Wang, Z., Issar, M., Betageri, G.V., 2014. Proliposomes as a drug delivery system to decrease the hepatic first-pass metabolism: Case study using a model drug. European Journal of Pharmaceutical Sciences 64, 26–36. https://doi.org/10.1016/j.ejps.2014.08.008
Yanyu, X., Yunmei, S., Zhipeng, C., Qineng, P., 2006. Preparation of silymarin proliposome: A new way to increase oral bioavailability of silymarin in beagle dogs. International Journal of Pharmaceutics 319, 162–168. https://doi.org/10.1016/j.ijpharm.2006.03.037
Youssef, S.F., Elnaggar, Y.S., Abdallah, O.Y., 2018. Elaboration of polymersomes versus conventional liposomes for improving oral bioavailability of the anticancer flutamide. Nanomedicine 13, 3025–3036. https://doi.org/10.2217/nnm-2018-0238
Zhao, M., Lee, S.H., Song, J.G., Kim, H.Y., Han, H.-K., 2018. Enhanced oral absorption of sorafenib via the layer-by-layer deposition of a pH-sensitive polymer and glycol chitosan on the liposome. International Journal of Pharmaceutics 544, 14–20. https://doi.org/10.1016/j.ijpharm.2018.04.020
