PLGA NANOPARTICLES
WHAT IS A NANOPARTICLE?
It falls in the general category of polymeric nanoparticles, which are uniform spherical nanostructures made of synthetic polymers. In this case, it is the linear copolymer poly(d,l-lactic-co-glycolic acid), usually referred to as PLGA. However less common, other polymers as poly(ε-caprolactone) or PCL; poly(ethylene glycol) or PEG, polyethyleneimine or PEI and poly(lactic acid) or PLA can be used as well.
The PLGA copolymer is synthesized via random ring-opening copolymerization of the two monomers lactic acid and glycolic acid by ester linkages. Different ratios of glycolic acid to lactic acid can be found (identified with the molar ratio) the most common are 50:50 and 75:25 lactic acid to glycolic acid. This is a determining factor in the final properties of the nanoparticle. On one side, lactic acid has a methyl group which makes it more hydrophobic (thus, it degrades slowly) and stiff. On the other side, glycolic acid is more hydrophilic (degrades faster) and is more flexible. The amount of each will determine the hydrophobicity of the polymer, the rate of degradation (that could go from months to years) and its amorphous structure. The poly(d,l-lactic-co-glycolic acid) polymer can be terminated in a carboxyl group (not end capped) or in an alkyl ester which protects against quick degradation.
Polymeric nanoparticles can be classified in two categories according to their morphology: nanocapsules and nanospheres. Nanocapsules contain an oily or aqueous core surrounded by a polymeric layer. On the contrary, nanospheres, consist of a solid continuous polymeric matrix. In either case, the drug is can be dissolved in the core (nanocapsules) or the polymeric network (nanospheres), or it can be adsorbed onto the nanoparticle surface.
PLGA nanoparticles are synthesized by emulsion-solvent evaporation methods. This is, preparing a single oil-in-water (o/w) emulsion for hydrophobic substances or double water-in-oil-in-water (w/o/w) emulsion for hydrophilic compounds. The emulsification is done using stirring or homogenization, emulsifiers and controlling the temperature which forms the nanoparticles. After that, the solvent is removed via evaporation or extraction and the PLGA drug-containing nanoparticles harden and can be collected.
WHAT IS A PLGA NANOPARTICLE DELIVERY SYSTEM?
Polymeric nanoparticles are excellent in vivo nanocarriers due to their biocompatibility (approved by the FDA) and their special suitability for oral administration of pharmaceuticals. They are made of biodegradable and biocompatible polymers or copolymers with a size in the range of nanometers, like PLGA – Poly (lactic-co-glycolic acid). Drugs can be encapsulated within the carrier, physically adsorbed onto, or chemically linked to the surface.
PLGA NANOPARTICLE PROPERTIES
FDA and EMA approved
Approved by the Food and Drug Administration (FDA) and the European Medicines Agency
Biodegradability and biocompatibility
The PLGA copolymer is naturally degraded by hydrolysis under biological and environmental conditions to lactic acid and glycolic acid, which are natural metabolic by-products. Then, they are cleared via the renal system.
Compound protection
Another challenge faced in the pharmaceutical industry is the degradation of the drug compound under light, pH or oxidative conditions. PLGA nanoparticles help maintaining the therapeutic activity and molecular integrity of the susceptible pharmaceuticals.
Hydrophilic and hydrophobic drugs
There are many protocols to prepare the polymeric nanoparticles which enable the loading of drugs with different polarities. Both hydrophobic and hydrosoluble drugs will be trapped in the polymeric matrix during the synthesis process and will be released in a controlled way.
PLGA VS PLGA-PEG VS PLGA-PEI
Bare PLGA nanoparticles are rarely used due their high hydrophobicity, short circulation times due to opsonization and low cellular uptake due to surface negative charge. Surface coatings with PEG or PEI are essential to ensure that the nanoparticles reach their target.
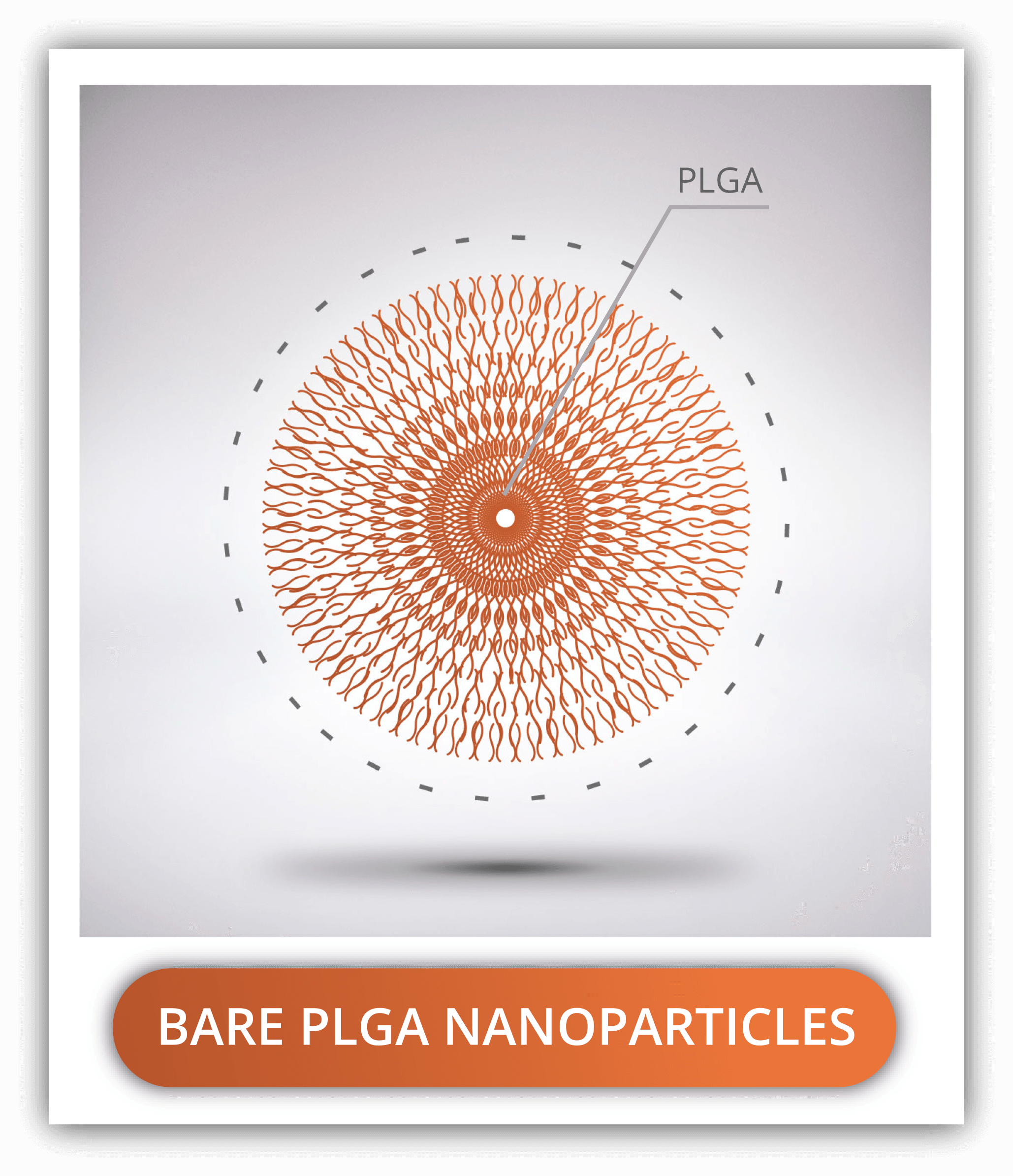
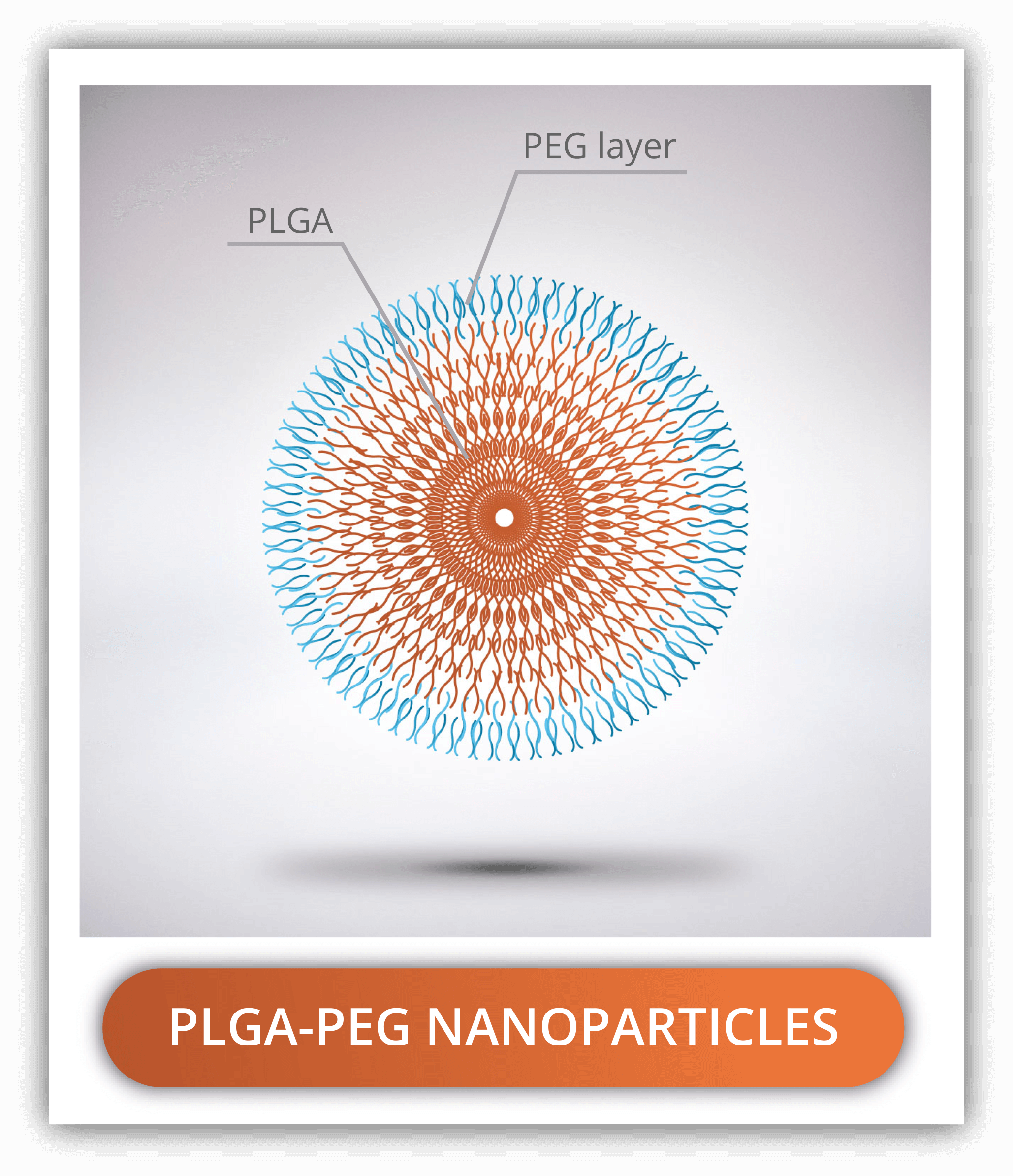
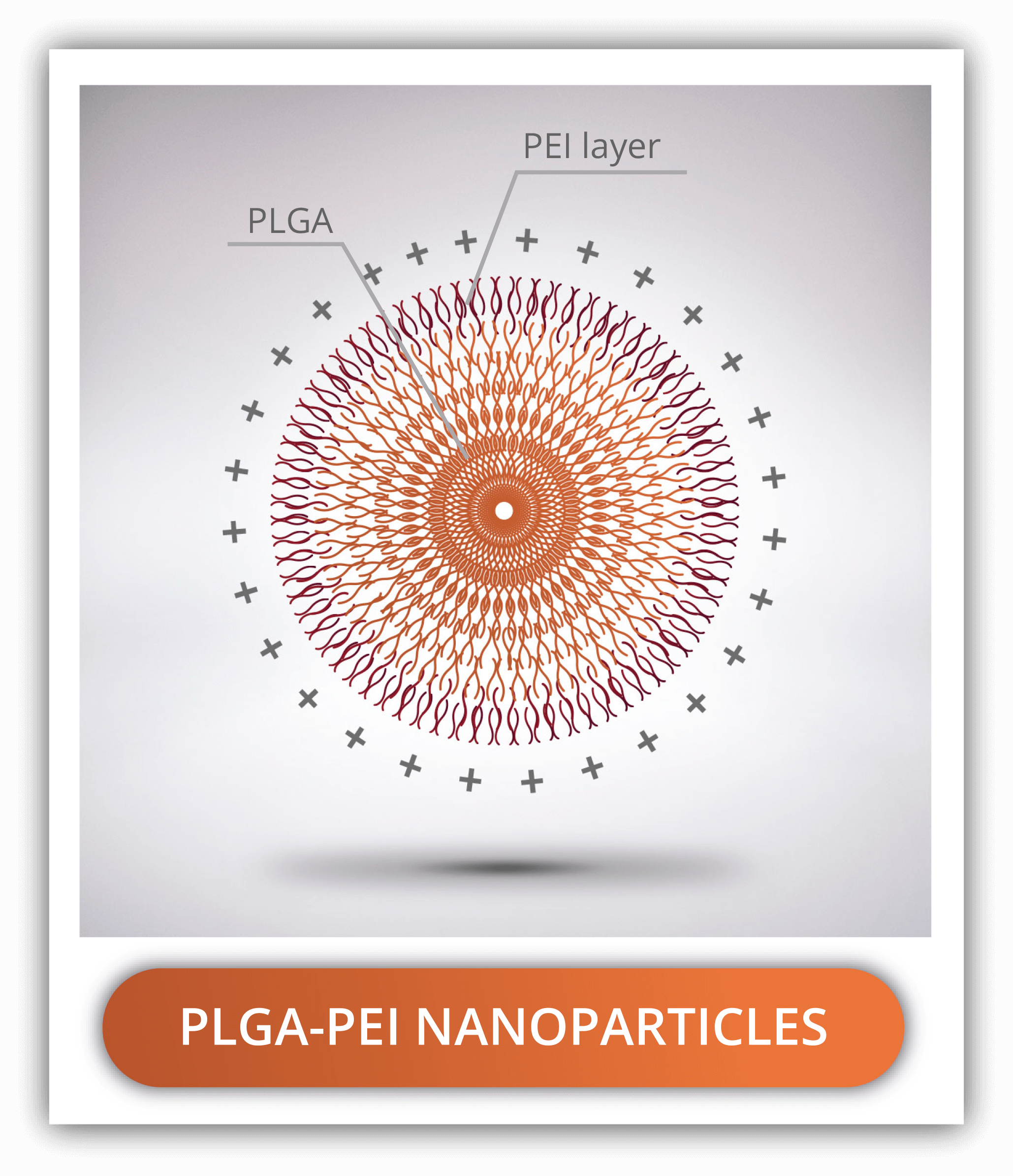
PLGA-PEG NANOPARTICLES
Poly(ethylene glycol) or PEG is the most common polymer used in combination with PLGA nanoparticles. It helps in reducing the nanoparticle aggregation, increasing solubility and stability over time, reducing immunogenicity and uptake from RES cells. All of this contribute to the increase in the circulation time. For these reasons, it is recommended for in vivo applications. While PLGA is naturally hydrophobic, PEG is the opposite, so the combination of both is amphiphilic, facilitating the emulsification process.
PEG-PLGA nanoparticles can be prepared by two methods: using of PLGA-PEG block copolymers or by adding a PEG coating after the preparation of the PLGA nanoparticles. The PEG-PLGA block copolymers are typically double or triple: PEG-PLGA, PEG-PLGA-PEG and PLGA-PEG-PLGA. As with the lactic acid : glycolic acid ratio, the ratio of PEG to PLGA in the in here will provide the molecule with different chemical properties. The higher the ratio (higher PEG content), the lower it is the molecular weight and the more soluble in aqueous media it is. On the contrary, if the ratio is low (higher PLGA content), the higher the molecular weight and the less hydro soluble the molecule is. For the second method, the PEG is linked to the PLGA nanoparticles by amide bonds between an amine terminated PEG derivative and a free carboxyl group of PLGA.
PLGA-PEI NANOPARTICLES
Polyethyleneimine, commonly referred to as PEI is a synthetic hydrosoluble polymer used to coat PLGA nanoparticles and confer them with a positive charge. This property makes PEI-PLGA nanoparticles suitable for the intracellular delivery of genetic material which is negatively charged. It has great affinity for lipopolysaccharides and cell membranes increasing circulation times of the nanoparticles.
PEI consists of the repeated monomer aziridine containing an amine group. PEI is available in a linear and several branched forms, the latest being the most positively charged. The formation of PLGA-PEI is the result of the electrostatic interaction of the amine group from the PEI and the carboxylic acid end from the PLGA.
The most cationic variants condense nucleic acids more and provides them with protection from nucleases. A PEI coating also favours the endosomal scape. Whereas the linear PEI only contains secondary amines, the branched PEI contains primary, secondary, and tertiary amine groups. The firsts are the ones that form the complexes with the nucleic acids, and the others play their role in disrupting the endosomal membrane due to their buffering effect.
USES AND APPLICATIONS
![]()
GENE
THERAPY
![]()
CANCER
TREATMENT
![]()
OCULAR DRUG
DELIVERY
![]()
DIABETES
TREATMENT
![]()
BRAIN DRUG
DELIVERY
![]()
VACCINES
![]()
BIOSENSORS
![]()
TISSUE
ENGINEERING
WHAT KIND OF DRUGS MAY BE DELIVERED BY PLGA NANOPARTICLES?

Proteins and enzymes

Genetic material

Lipophilic compounds
Pharmaceutical drugs
Find below some examples of drugs that have been encapsulated in PLGA nanoparticles with their specific applications.
| TYPE OF PLGA NANOPARTICLES | ENCAPSULATED COMPOUND | APPLICATION |
| PLGA | Pronaprofeno | Cataract surgery |
| PLGA | SPIONS, QDs and Busulfan | Breast cancer |
| PLGA | Moxifloxacin | Corneal infection |
| PLGA | Insulin | Diabetes |
| PLGA | Ciprofloxacin | Bone implants |
| Cationic PLGA:PEI | Plasmid DNA | Lymhoma |
| Cationic PLGA:PEI | Plasmid DNA | Bacterial infection |
| Cationic PLGA:PEI | Plasmid DNA | Prostate cancer |
| Cationic PLGA:Chitosan | Forskolina | Glaucoma |
| Cationic PLGA-Chitosan | Plasmid DNA | Viral infection |
| PLGA:PEG | Curcumin | Breast cancer |
| PLGA:PEG | Taxol | Cancer |
| PLGA:PEG:CHITOSAN | Moxifloxacin | Tuberculosis |
| PLGA:PEG | Cetuximab | Lung cancer |
| PLGA-PEG | Camptothecin | Sarcoma Solid Tumor |
| HA-PLGA | Docetaxel | Lung cancer |
| HA-PLGA | Doxorubicin and Irinotecan | Breast cancer |
| HA-PLGA | Camptothecin and Curcumin | Colon cancer |
| antiCD64-PLGA | methotrexate and SPIONs | Rheumatoid arthritis |
| Lactoferrin-PEG-PLGA | Urocortin | Parkinson |
| mAb-PLGA | Docetaxel | Lung cancer |
HOW DO PLGA NANOPARTICLES PROVIDE A WAY OF TARGETING DRUGS TO A SPECIFIC TISSUE?
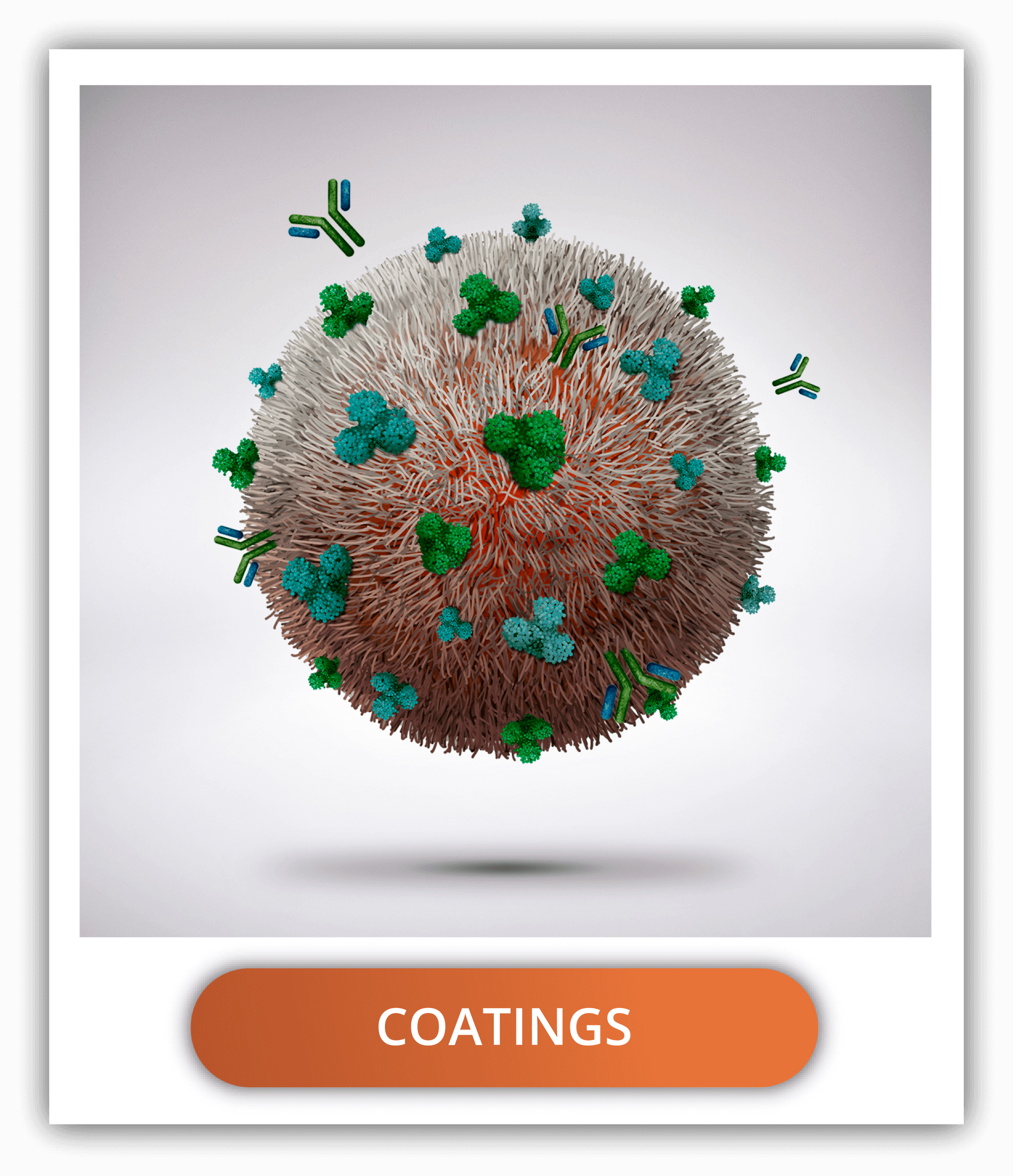
The delivery in specific tissues with PLGA nanoparticles can be tuned using surface functionalization, which is the combination of coatings and targeting ligands.
The most common coatings are PEG and PEI as mentioned before, but also chitosan, hyaluronic acid and human serum albumin are other hydrophilic alternatives to increase the circulation time and reduce the degradation of PLGA.
Functionalization of PLGA nanoparticles with ligands to target certain cells can be done through covalent or non-covalent binding. The covalent binding is done by means of union to the carboxyl end groups of the uncapped PLGA. Non-covalent binding is done through the electrostatic interactions between the uncapped and negatively charged PLGA and positively charged molecules or by exploiting hydrophobic interactions.
- Biotin-NH2 is one of the most versatile ligands to add to the PLGA nanoparticles. Its high affinity to avidin and streptavidin allows the conjugation of many other compounds like antibodies. Targeting antibodies are a common strategy to reach certain cell types. For example, the anticytokeratin monoclonal IgG has shown useful in targeting breast cancer cells or antiCD-40 that targets dendritic cells.
- The decoration of nanoparticles with some compounds has been inspired in the mechanisms of infectious agents. For example, the group of lectin proteins, can bind to glycosylated components in cell membranes (CLRs: C-type lectin receptors), promoting the internalization of the nanoparticles. More than 60 CLRs have been identified in humans. One of those is the mannose receptor present in dendritic cells and macrophages, as well as some cancer cells, which can bind to mannan functionalized nanoparticles.
- Transferrin is another targeting ligand that allows the selective uptake from cancerous cells, which overexpress the receptors for this compound, as demonstrated with glioma cells.

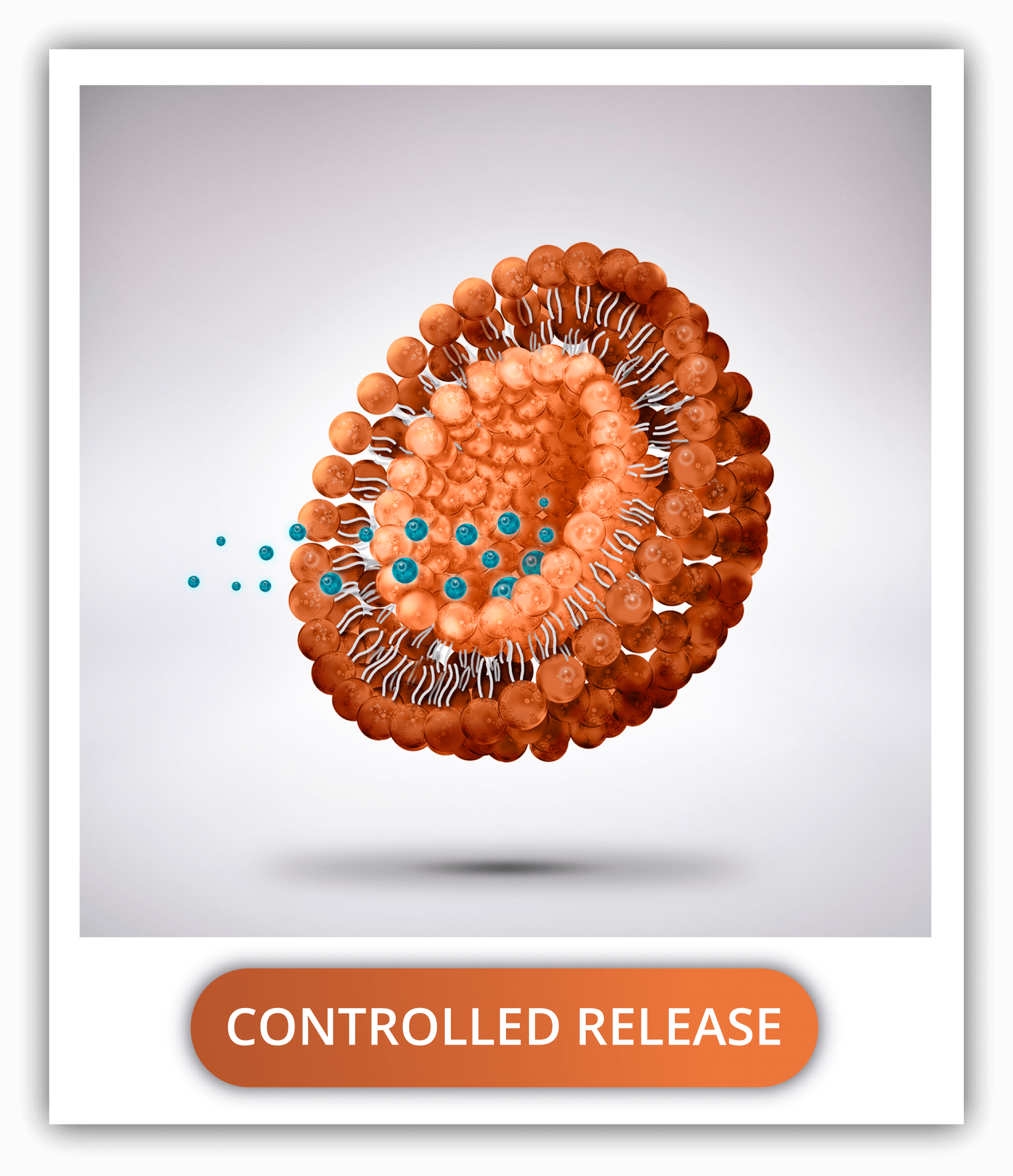
HOW CAN PLGA NANOPARTICLES CONTROL THE RELEASE RATE OF A DRUG?
PLGA degradation creates channels through which the drug escapes and it’s released into its surroundings. Fortunately, this speed of degradation depends greatly on the nanoparticle physical properties and composition as well as the production methods.
Some factors are the ratio of lactic acid to glycolic acid (determining the hydrophobicity and molecular weight), the size (bigger nanoparticles degrade faster, and some diameters are greatly cleared up by the RES cells or the kidneys), the monomeric sequence (random being degraded faster), termination sequence (uncapped and acid capped faster degradation compared to ester capped), media pH (acidic medium faster degradation), temperature (directly proportional) and surface area; among many.
By considering all the settings in the design and conducting an extensive characterization of the nanoparticles, the delivery rate of the drug can be easily controlled.
BUY PLGA NANOPARTICLES FOR DRUG DELIVERY
Do you have a drug delivery project compatible with plga nanoparticles? Get in contact with us and tell us a little bit about your project. We will assess you and prepare a PLGA nanoparticle design that ensures your success.
