DELIVERY STRATEGIES
The are several strategies to direct compound release. These strategies allow the pharmaceutical to reach the site of action effectively with minimal loss. They can also be combined to act synergically and create a unique approach that ensures the success of the therapy or diagnostic.
PARAMETERS OF INTEREST
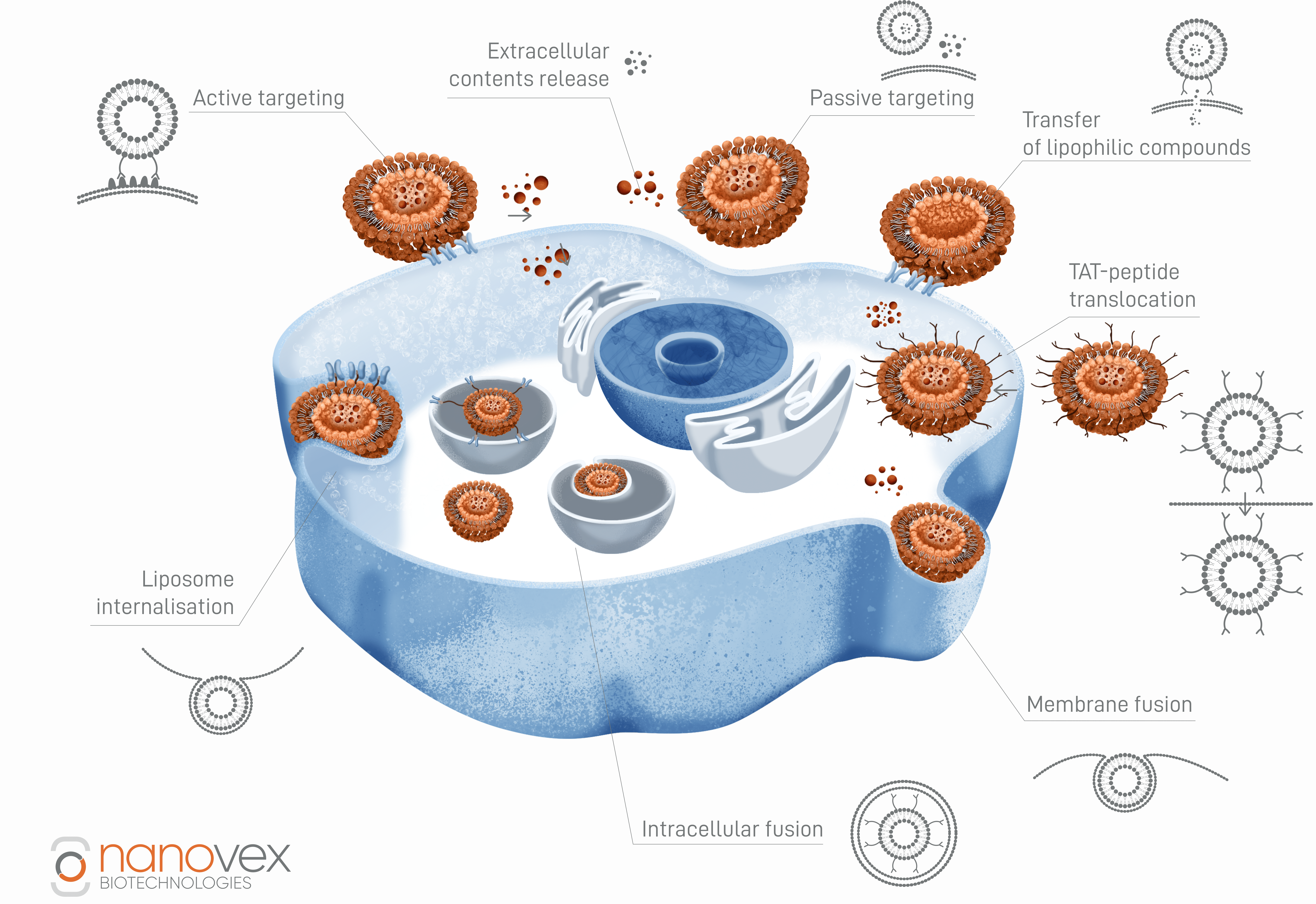
Delivery of drug carriers to specific cells by recognition of surface biomarkers is an advantageous approach. Not only does it minimize toxicity and adverse reactions, but it also helps in overcoming the limitations of some previous therapies by releasing the drug through different internalization pathways.
Since most cells are not able to take up naked nanoparticles (except for reticuloendothelial cells and macrophages) the addition of targeting ligands is necessary to reach certain cells or organelles. Those ligands bind to cell/organelle unique receptors that can be either protein, carbohydrate or lipid based.
After that, they can be internalized through several paths to deliver the drug in the cells, which will highly depend on the stability of the compound and the aim of the delivery. For example, in some cases biological materials introduced into cells via endocytosis lose their biological activity due to contact with acidic media. In these cases, the use of fusogenic liposomes is an alternative to the release of drugs like in gene therapy. The high efficiency of this system is attributed to the fact that materials introduced by membrane fusion can escape lysosomal degradation and reach the cytoplasm intact.
Applications of targeted delivery strategies
CLICK TO DISCOVER MORE!
![]()
INTRACELLULAR
DELIVERY
![]()
CANCER
TREATMENT
![]()
OCULAR DRUG
DELIVERY
![]()
BRAIN DRUG
DELIVERY
![]()
DIABETES
Intracellular delivery
Delivery of the encapsulated compound inside the cell can be done through various routes.
Some examples are transfer of lipophilic compounds, intracellular fusion, liposome internalization, TAT-peptide translocation, and membrane fusion.
Cancer treatment
Tumor masses present what is referred to as enhanced permeability and retention effect (EPR). They develop its own vasculature that enable particles up to 200 nm to leak to the cancer site. However, in many cases, the addition of a ligand is also used to enhance the delivery in those cancer cell types. Some examples are monoclonal antibodies, peptides, folate, or transferrin.
Brain targeting
Different targeting ligands such as antibodies and aptamers can be used to enhance drug accumulation in the brain. Another example of ligands used could be glutathione, methoxy-XO4, insulin, thiamine, peptides, and transferrin.
Ocular delivery
Studies have shown that addition of RGD peptide ligand onto the surface of the nanocarrier targets corneal epithelial cells. This enables both the endocytosis of the nanovehicle as well as the specific release of the drug in the ocular tissue.
Diabetes
Although Type 1 diabetes has always been considered an irreversible autoimmune disease, some cell types, like Treg, are able to restore immune tolerance and suppression of the disease development. Liposomes targeted towards Treg cells have successfully been used to release therapeutic levels of immunomodulators, improving the autoimmune response.
Controlled release intends to deliver the therapeutic compound in the chosen region and in the required period. Both the release rate and where it takes place can be controlled.
Release rate
Different parameters must be adjusted, the composition being the main one. The release could be systematically or locally, and the rate could be very fast, being able to release all the encapsulated compound in a few minutes or, conversely, very low, taking place a sustained release over time for several weeks.
Stimuli-responsive systems
Stimuli-responsive systems are able to control drug biodistribution in response to specific stimuli, either those found in the local environment of the target place (changes in pH, enzyme concentration or redox gradients) and those that are triggered by an external physical stimulus such as heat, ultrasound or light.
Applications of controlled release delivery strategies
CLICK TO DISCOVER MORE!
![]()
pH
SENSITIVE
![]()
ENZYME-RESPONSIVE
![]()
REDOX
RESPONSIVE
![]()
HERMAL
RESPONSIVE
pH sensitive: Acidification of the nanocarrier environment triggers the release of its contents. It has been shown to be a great technique to target cancer cells since they have lower pH values than normal. Additionally, it can specifically reach organelles inside the cell like endosomes or lysosomes which pH is more acidic.
Enzyme-responsive: The destabilization of the nanocapsule membrane in the presence of a certain enzyme therefore releasing the cargo helps in accurately drug delivery. Enzymes that are overexpressed in the target tissue are great triggers for enzyme-responsive nanocarriers. Some examples could be phospholipase, matrix metalloproteinases, urokinase plasminogen activator, and many others that are involved in inflammatory and cardiovascular diseases as well as different cancers.
Redox responsive: The higher concentration inside cancer cells of glutathione generates a redox gradient that can be used to trigger the release of pharmaceuticals in its destiny.
Thermal sensitive liposomes: This strategy is especially useful when drug delivery is used in combination with local hyperthermia for cancer treatments. This way, when the heat treatment starts, the pharmaceutical is released which results in superior targeting and treatment efficiency.
Nanocarriers have enabled the controlled release of many compounds which are instable or cannot enter the cells on their own. However, their use in vivo is hindered by the interference from blood cells which prevent the release of the carried compound in the target tissue.
Modification of nanovesicles and nanoparticles surfaces with polymers and biomaterials, among others, provides nanosystems with increased steric stability and resistance. Additionally, it allows in vivo delivery strategies like long-circulating particles/vesicles or resistance to gastric conditions for intestinal release. Although there are many possibilities regarding nanoparticle or nanovesicle coatings, excellent examples are chitosan and PEG.
Chitosan
Chitosan is a great option to consider when coating liposomes. It forms polyelectrolyte layers by the interaction of chitosan (positive) with the surface of the liposomes (negative). That causes the nanovesicles to be more stable and mucoadhesive. Not only that but also it shows great biocompatible properties such as biodegradability and non-toxicity.
Other hydrophilic or glycolipids
Other hydrophilic polymers or glycolipids like PEG or GM1 help in removing unwanted interactions. Those have a flexible chain that occupy the space immediately adjacent to the nanovesicle or nanoparticle surface. As a consequence, it excludes other macromolecules from this space. Blood plasma opsonins cannot bind anymore to the nanocarrier, thus avoiding macrophage unspecific interactions.
Applications of coatings as delivery strategies
CLICK TO DISCOVER MORE!
![]()
MOLECULAR
THERAPY
![]()
WOUND
HEALING
![]()
OCULAR DRUG
DELIVERY
![]()
CANCER
TREATMENT
![]()
BRAIN DRUG
DELIVERY
![]()
INTRANASAL
ADMINISTRATION
Biomedical imaging as well as cell tracking play a key role in understanding structural as well as functional biological systems. However useful, traditional methods like bioluminescence and fluorescence imaging present some challenges that can be overcome with the use of nanotechnology.
Liposomes and polymeric nanovesicles, are of great interest due to their capacity to deliver a specific drug to its target while monitoring and tracking the location of the nanosystem in real time, and in vivo. This holds great potential to study the distribution as well as the bioaccumulation of the drug which is an indicator of efficiency and possible toxicity.
Additionally, a great variety of nanoparticles have already been developed and even used in clinical trials up to phase 4 for in vivo imaging, including spions, silver nanoparticles and CdS/ZnS quantum dots.
While this technology has been used to enhance different imaging modalities, it stands out in the case of magnetic resonance imaging as excellent contrasting agents. Nanoparticle cell tracking has also been reported with Magnetic Particle Imaging (MPI), fluorescence imaging, nuclear and photoacoustic imaging.
Additionally, nanoparticles can be multi-functional, allowing for different imaging methods to be used simultaneously and enabling more information to be obtained. Further functionalization can also help distinguish subtypes of tissues as in the case of tumors.
Applications of imaging and tracking as delivery strategies
CLICK TO DISCOVER MORE!
![]()
STEM CELL
TRACKING
![]()
SENSING
MOLECULAR
EVENTS
![]()
DIAGNOSTIC
![]()
THERANOSTICS